SARS-CoV-2 Antigen Rapid Test Kit (Fluorescence Immunochromatography) gets special approval by the German Federal Institute for Drugs and Medical Devices (BfArM),which is responsible for licensing and registering products as proof of safety and efficacy. This test is now included in the Antigen List on BfArM as shownin the screenshot below.
https://antigentest.bfarm.de/ords/f?p=110:100:8176733948529:::::&tz=8:00
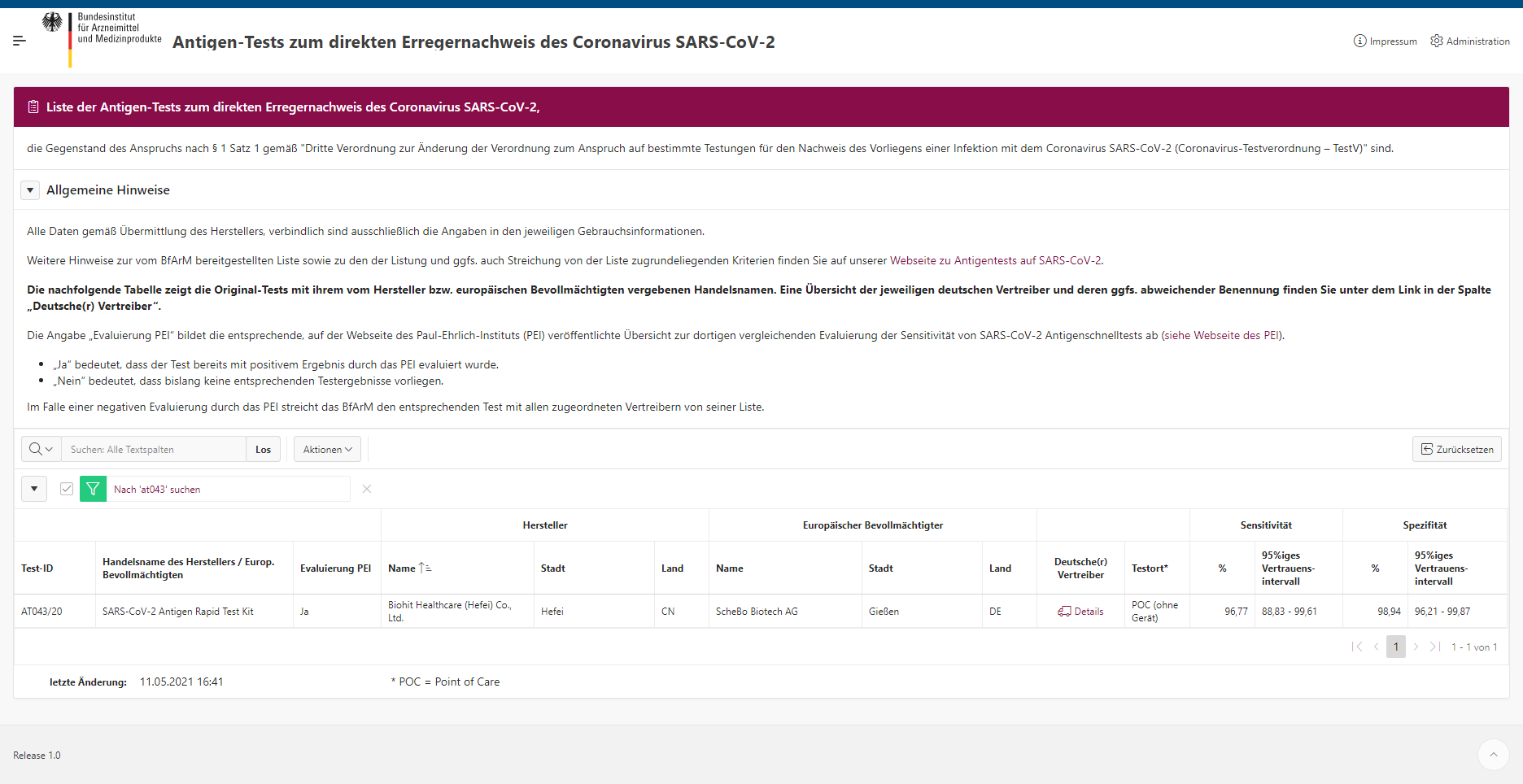
Recently, this fluorescence test also passed the evaluation of Paul Ehrlich Institute (PEI), which is a German federal agency and medical regulatory body. Sample tests being evaluated, the performance of our test proves to be consistent with the claim on the IFU and study reports.
This SARS-CoV-2 Antigen Rapid Test Kit (Fluorescence Immunochromatography) is now legally marketed in Europe. It is fast, reliable, affordable and easy to use with excellent performance. With the approval of BfArM and PEI, we are looking forward to tap into the German market and to contribute our part in fighting against the pandemic.

